NEET Chemistry


NEET Chemistry is a vital and high-scoring section of the National Eligibility cum Entrance Test (NEET), the premier medical entrance exam in India for admission to MBBS, BDS, and other medical courses.
It is divided into three branches:
Physical Chemistry
Organic Chemistry
Inorganic Chemistry
Physical Chemistry focuses on concepts like atomic structure, thermodynamics, chemical kinetics, and equilibrium, requiring both theoretical understanding and numerical problem-solving skills.
Organic Chemistry emphasizes reaction mechanisms, functional groups, and biomolecules, demanding logical reasoning and memorization of key reactions.
Inorganic Chemistry covers the periodic table, coordination compounds, and chemical bonding, often relying on factual knowledge and trends.
The questions are primarily based on the NCERT curriculum, making it essential for students to thoroughly study and practice from NCERT textbooks. NEET Chemistry is considered relatively easier compared to Physics and Biology, but it requires consistent practice, conceptual clarity, and time management to secure a high score. A strong performance in Chemistry can significantly boost the overall NEET rank, making it a crucial subject for medical aspirants.
Pattern of Studies
● Interactive Classes
● Home Assignments
● Class Notes
● Periodic Class Tests
● Student's Monthly Report
● One to One Attention
● Doubt Classes
● Syllabus Revision
Report Analysis Parameters
● Tests Marks
● Attendance
● Class Discipline
● Class Response
● Home Assignments
● Student's Comparison with Class
● Last 3 Months Performance
● Special Remarks
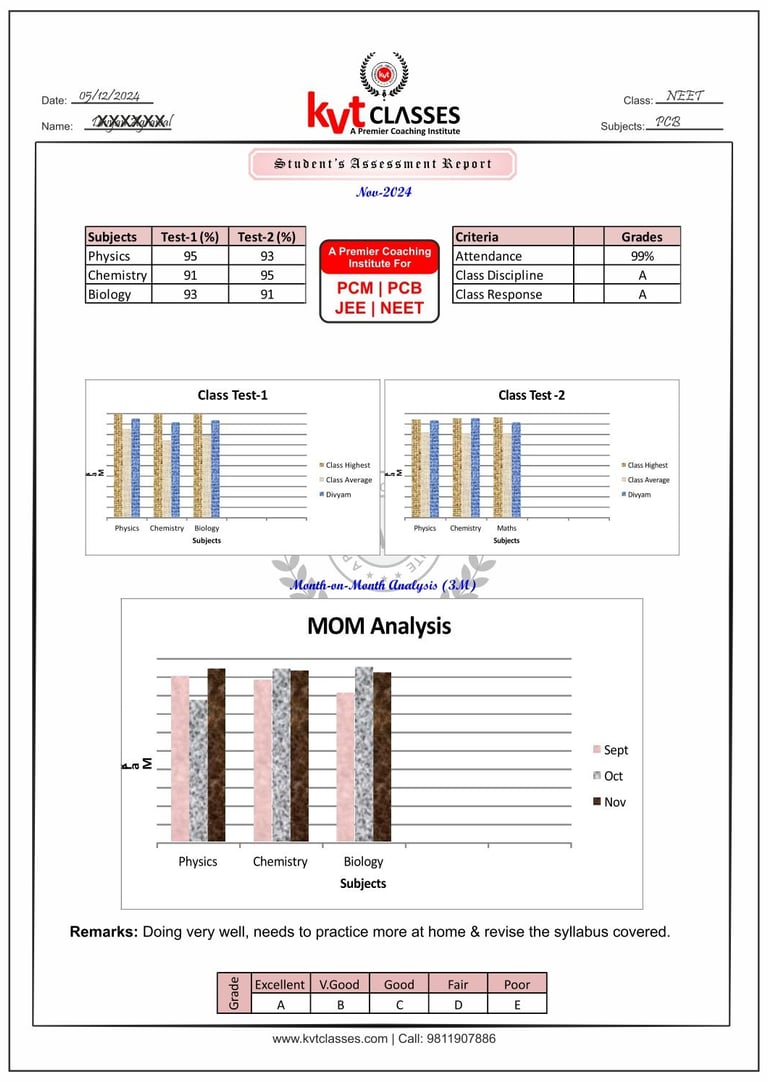
Student's Sample Report
Class Tests
● Two tests per subject in a month
● Test schedule on Saturday in extra hours
● Result within 1 week of test conduct
● Test discussion on result day
● Mistakes rectification briefing
Conduct of Classes
● On board explanation with notes
● Make Concept clear with practical examples
● Interactive class sessions
● Home work / Assignment at the end of the class
● Home work / Assignment check in the next class
● Quick recap of previous class in the next class
● Two tests per month/subject on Saturdays
● Class duration 1.5 Hr/sub alternate days (4.5Hrs./wk)
● Extra classes provided when needed
Classes Schedule 2024-25
JEE for Class XI / XII / Dropper Batch
Note: Class Tests will be conducted on Saturday in extra hours


Preparation Tips for NEET
National Entrance cum Eligibility Test (NEET) requires a well-structured plan, dedication, and consistent effort. Here are some preparation tips to help you excel in NEET:
1. Understand the Exam Pattern and Syllabus
Understand the exam pattern (number of questions, marking scheme, etc) for NEET (UG).
Syllabus: Thoroughly go through the NCERT syllabus for Class 11 and 12, as it forms the foundation.
2. Create a Study Plan
Daily Schedule: Allocate time for each subject daily.
Weekly Goals: Set achievable targets for each week.
Revision Time: Dedicate time for regular revision.
Mock Tests: Include regular practice tests to assess your progress.
3. Focus on Conceptual Clarity
Physics: Understand concepts and practice numerical problems.
Chemistry: Focus on both theory (Inorganic) and problem-solving (Physical and Organic).
Biology: Emphasize diagrams, definitions, and conceptual understanding.
4. Use the Right Study Material
Books:
Physics: HC Verma, DC Pandey
Chemistry: NCERT, OP Tandon, MS Chauhan
Biology: NCERT, Trueman’s Biology, GRB Bathla Publications
5. Practice Previous Years’ Papers
Solve at least 10 years NEET papers.
Analyze your mistakes and work on weak areas.
6. Take Mock Tests
Simulate exam conditions to improve time management.
Analyze your performance and identify areas for improvement.
7. Strengthen Your Basics
Focus on NCERT books for Chemistry and Biology.
Build a strong foundation in Physics concepts.
8. Time Management
Allocate time wisely during the exam.
Practice solving questions within a set time limit.
9. Stay Healthy and Positive
Sleep: Get 6-8 hours of sleep daily.
Diet: Eat healthy and stay hydrated.
Exercise: Take short breaks and engage in physical activities to stay fresh.
10. Stay Consistent and Motivated
Avoid procrastination.
Stay focused on your goal and maintain a positive mindset.
11. Seek Help When Needed
Clear doubts with teachers, mentors because concept clarity is the key.
KVT Classes help you clear every concept, but self-study is equally important.
12. Revision is Key
Regularly revise formulas, concepts, and important topics.
Make short notes for quick revision.
13. Avoid Common Mistakes
Don’t ignore any subject or topic.
Don’t rely solely on shortcuts; focus on understanding.
Avoid last-minute cramming.
14. Stay Updated
Keep track of exam dates, admit cards, and any changes in the exam pattern.
PHYSICAL CHEMISTRY
UNIT I: SOME BASIC CONCEPTS IN CHEMISTRY
Matter and its nature, Dalton's atomic theory, Concept of atom, molecule, element and compound, Laws of chemical combination, Atomic and molecular masses, mole concept, molar mass, percentage composition, empirical and molecular formulae, Chemical equations and stoichiometry.
UNIT 2: ATOMIC STRUCTURE
Nature of electromagnetic radiation, photoelectric effect, spectrum of the hydrogen atom, Bohr model of a hydrogen atom - its postulates, derivation of the relations for the energy of the electron and radii of the different orbits, limitations of Bohr's model, dual nature of matter, de Broglie's relationship, Heisenberg uncertainty principle, elementary ideas of quantum mechanics, the quantum mechanical model of the atom and its important features, concept of atomic orbitals as one-electron wave functions, variation of and 2 with r for 1s and 2s orbitals, various quantum numbers (principal, angular momentum and magnetic quantum numbers) and their significance, shapes of s, p and d - orbitals, electron spin and spin quantum number, rules for filling electrons in orbitals – Aufbau principle, Pauli's exclusion principle and Hund's rule, electronic configuration of elements and extra stability of half-filled and completely filled orbitals.
UNIT 3: CHEMICAL BONDING AND MOLECULAR STRUCTURE
Kossel-Lewis approach to chemical bond formation, the concept of ionic and covalent bonds.
Ionic Bonding: Formation of ionic bonds, factors affecting the formation of ionic bonds; calculation of lattice enthalpy.
Covalent Bonding: Concept of electronegativity, Fajan’s rule, dipole moment, Valence Shell Electron Pair Repulsion (VSEPR ) theory and shapes of simple molecules.
Quantum mechanical approach to covalent bonding: Valence bond theory - its important features, the concept of hybridization involving s, p and d orbitals, resonance.
Molecular Orbital Theory - Its important features, LCAOs, types of molecular orbitals (bonding, antibonding), sigma and pi-bonds, molecular orbital electronic configurations of homonuclear diatomic molecules, the concept of bond order, bond length and bond energy.
Elementary idea of metallic bonding, hydrogen bonding and its applications.
UNIT 4: CHEMICAL THERMODYNAMICS
Fundamentals of thermodynamics: System and surroundings, extensive and intensive properties, state functions, entropy, types of processes.
The first law of thermodynamics - Concept of work, heat, internal energy and enthalpy, heat capacity, molar heat capacity, Hess’s law of constant heat summation, Enthalpies of bond dissociation, combustion, formation, atomization, sublimation, phase transition, hydration, ionization and solution.
The second law of thermodynamics - Spontaneity of processes, of the universe and of the system as criteria for spontaneity. (Standard Gibbs energy change) and equilibrium constant.
UNIT 5: SOLUTIONS
Different methods for expressing the concentration of solution - molality, molarity, mole fraction, percentage (by volume and mass both), the vapour pressure of solutions and Raoult's Law - Ideal and nonideal solutions, vapour pressure - composition, plots for ideal and non- ideal solutions, Colligative properties of dilute solutions - a relative lowering of vapour pressure, depression of freezing point, the elevation of boiling point and osmotic pressure, determination of molecular mass using colligative properties, abnormal value of molar mass, van’t Hoff factor and its significance.
UNIT 6: EQUILIBRIUM
Meaning of equilibrium is the concept of dynamic equilibrium.
Equilibria involving physical processes: Solid-liquid, liquid-gas, gas-gas and solid-gas equilibria, Henry's law. General characteristics of equilibrium involving physical processes.
Equilibrium involving chemical processes: Law of chemical equilibrium, equilibrium constants (Kp and Kc) and their significance, the significance of chemical equilibrium, factors affecting equilibrium concentration, pressure, temperature, the effect of catalyst, Le Chatelier’s principle.
Ionic equilibrium: Weak and strong electrolytes, ionization of electrolytes, various concepts of acids and bases (Arrhenius, Bronsted - Lowry and Lewis) and their ionization, acid-base equilibria (including multistage ionization) and ionization constants, ionization of water, pH scale, common ion effect, hydrolysis of salts and pH of their solutions, the solubility of sparingly soluble salts, solubility products and buffer solutions.
UNIT 7: REDOX REACTIONS AND ELECTROCHEMISTRY
Electronic concepts of oxidation and reduction, redox reactions, oxidation number, rules for assigning oxidation number and balancing of redox reactions.
Electrolytic and metallic conduction, conductance in electrolytic solutions, molar conductivities and their variation with concentration, Kohlrausch’s law and its applications.
Electrochemical cells - Electrolytic and Galvanic cells, different types of electrodes, electrode potentials including standard electrode potential, half-cell and cell reactions, emf of a Galvanic cell and its measurement, Nernst equation and its applications, relationship between cell potential and Gibbs' energy change, dry cell and lead accumulator, fuel cells.
UNIT 8: CHEMICAL KINETICS
Rate of a chemical reaction, factors affecting the rate of reactions: concentration, temperature, pressure and catalyst, elementary and complex reactions, order and molecularity of reactions, rate law, rate constant and its units, differential and integral forms of zero and first-order reactions, their characteristics and half-lives, the effect of temperature on the rate of reactions, Arrhenius theory, activation energy and its calculation, collision theory of bi-molecular gaseous reactions (no derivation).
INORGANIC CHEMISTRY
UNIT 9: CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
Modern periodic law and present form of the periodic table, s, p. d and f block elements, periodic trends in properties of elements atomic and ionic radii, ionization enthalpy, electron gain enthalpy, valence, oxidation states and chemical reactivity.
UNIT 10: p- BLOCK ELEMENTS
Group -13 to Group 18 Elements
General Introduction: Electronic configuration and general trends in physical and chemical properties of elements across the periods and down the groups, unique behaviour of the first element in each group.
UNIT 11: d - and f- BLOCK ELEMENTS
Transition Elements - General introduction, electronic configuration, occurrence and characteristics, general trends in properties of the first-row transition elements - physical properties, ionization enthalpy, oxidation states, atomic radii, colour, catalytic behaviour, magnetic properties, complex formation, interstitial compounds, alloy formation, preparation, properties and uses of K2Cr2O7 and KMnO4.
Inner Transition Elements
Lanthanoids - Electronic configuration, oxidation states and Lanthanoid contraction.
Actinoids - Electronic configuration and oxidation states.
UNIT 12: COORDINATION COMPOUNDS
Introduction to coordination compounds. Werner's theory, ligands, coordination number, denticity, chelation, IUPAC nomenclature of mononuclear co-ordination compounds, isomerism, Bonding: Valence bond approach and basic ideas of Crystal field theory, colour and magnetic properties, importance of coordination compounds (in qualitative analysis, extraction of metals and in biological systems).
ORGANIC CHEMISTRY
UNIT 13: PURIFICATION AND CHARACTERISATION OF ORGANIC COMPOUNDS
Purification - Crystallization, sublimation, distillation, differential extraction and chromatography - principles and their applications.
Qualitative analysis - Detection of nitrogen, sulphur, phosphorus and halogens.
Quantitative analysis (basic principles only) - Estimation of carbon, hydrogen, nitrogen, halogens, sulphur and phosphorus.
Calculations of empirical formulae and molecular formulae, numerical problems in organic quantitative analysis,
UNIT 14: SOME BASIC PRINCIPLES OF ORGANIC CHEMISTRY
Tetravalency of carbon, shapes of simple molecules - hybridization (s and p): classification of organic compounds based on functional groups and those containing halogens, oxygen, nitrogen and sulphur, homologous series: Isomerism - structural and stereoisomerism.
Nomenclature (Trivial and IUPAC)
Covalent bond fission - Homolytic and heterolytic, free radicals, carbocations and carbanions, stability of carbocations and free radicals, electrophiles and nucleophiles.
Electronic displacement in a covalent bond
- Inductive effect, electromeric effect, resonance and hyperconjugation.
Common types of organic reactions- Substitution, addition, elimination and rearrangement.
UNITS 15: HYDROCARBONS
Classification, isomerism, IUPAC nomenclature, general methods of preparation, properties and reactions.
Alkanes - Conformations: Sawhorse and Newman projections (of ethane), mechanism of halogenation of alkanes.
Alkenes - Geometrical isomerism, mechanism of electrophilic addition, addition of hydrogen, halogens, water, hydrogen halides (Markownikoffs and peroxide effect), Ozonolysis and polymerization.
Alkynes - Acidic character, addition of hydrogen, halogens, water and hydrogen halides, polymerization.
Aromatic hydrocarbons - Nomenclature, benzene - structure and aromaticity, mechanism of electrophilic substitution, halogenation, nitration.
Friedel-Craft's alkylation and acylation, directive influence of the functional group in mono- substituted benzene.
UNIT 16: ORGANIC COMPOUNDS CONTAINING HALOGENS
General methods of preparation, properties and reactions, nature of C-X bond, mechanisms of substitution reactions.
Uses, environmental effects of chloroform, iodoform, freons and DDT.
UNIT 17: ORGANIC COMPOUNDS CONTAINING OXYGEN
General methods of preparation, properties, reactions and uses.
ALCOHOLS, PHENOLS AND ETHERS
Alcohols: Identification of primary, secondary and tertiary alcohols, mechanism of dehydration.
Phenols: Acidic nature, electrophilic substitution reactions, halogenation, nitration and sulphonation, Reimer - Tiemann reaction.
Ethers: Structure.
Aldehyde and Ketones: Nature of carbonyl group, nucleophilic addition to >C=O group, relative reactivities of aldehydes and ketones, important reactions such as - Nucleophilic addition reactions (addition of HCN, NH3 and its derivatives), Grignard reagent, oxidation, reduction (Wolf Kishner and Clemmensen), the acidity of -hydrogen. Aldol condensation, Cannizzaro reaction, Haloform reaction, chemical tests to distinguish between aldehydes and ketones.
Carboxylic Acids: Acidic strength and factors affecting it.
UNIT 18: ORGANIC COMPOUNDS CONTAINING NITROGEN
General methods of preparation, properties, reactions and uses.
Amines: Nomenclature, classification, structure, basic character and identification of primary, secondary and tertiary amines and their basic character.
Diazonium Salts: Importance in synthetic organic chemistry.
UNIT 19: BIOMOLECULES
General introduction and importance of biomolecules.
CARBOHYDRATES – Classification, aldoses and ketoses, monosaccharides (glucose and fructose) and constituent monosaccharides of oligosaccharides (sucrose, lactose and maltose).
PROTEINS - Elementary idea of -amino acids, peptide bond, polypeptides, proteins: primary, secondary, tertiary and quaternary structure (qualitative idea only), denaturation of proteins, enzymes.
VITAMINS – Classification and functions.
NUCLEIC ACIDS – Chemical constitution of DNA and RNA, biological functions of nucleic acids.
Hormones (General introduction)
UNIT 20: PRINCIPLES RELATED TO PRACTICAL CHEMISTRY
Detection of extra elements (Nitrogen, sulphur, halogens) in organic compounds, detection of the following functional groups, hydroxyl (alcoholic and phenolic), carbonyl (aldehyde and ketones) carboxyl and amino groups in organic compounds.
1. The chemistry involved in the preparation of the following:
Inorganic compounds, Mohr’s salt, potash alum.
Organic compounds: Acetanilide, p-nitro acetanilide, aniline yellow, iodoform.
2. The chemistry involved in the titrimetric exercises – acids, bases and the use of indicators, oxalic-acid vs KMnO4, Mohr’s salt vs KMnO4
3. Chemical principles involved in the qualitative salt analysis:
Cations – Pb2+, Cu2+, Al3+, Fe3+, Zn2+, Ni2+, Ca2+, Ba2+, Mg2+, NH4+
Anions- CO32−, S2-, SO42−, NO3-, NO2-, Cl-, Br-, I- ( Insoluble salts excluded).
Chemical principles involved in the following experiments:
1. Enthalpy of solution of CuSO4
2. Enthalpy of neutralization of strong acid and strong base.
3. Preparation of lyophilic and lyophobic sols.
4. Kinetic study of the reaction of iodide ions with hydrogen peroxide at room temperature.
