Physics | Chemistry | Biology
480+ Hrs. of Extensive Studies for Physics, Chemistry and Biology with aprox. 160+ Hrs. devoted on each subject. Along with this Class Tests, Assignments, Doubt Sessions, Syllabus Revision in the end also included. Our expert faculties are Masters Qualified in their specialized subject and having more than 10+ years of experience and expertise to provide in depth knowledge for the subject concerned.
Senior Secondary stage of school education is a stage of transition from general education to discipline-based focus on curriculum. The present updated syllabus keeps in view the rigor and depth of disciplinary approach as well as the comprehension level of learners. Due care has also been taken that the syllabus is comparable to the international standards.
Pattern of Studies
● Interactive Classes
● Home Assignments
● Class Notes
● Periodic Class Tests
● Student's Monthly Report
● One to One Attention
● Doubt Classes
● Syllabus Revision
Report Analysis Parameters
● Tests Marks
● Attendance
● Class Discipline
● Class Response
● Home Assignments
● Student's Comparison with Class
● Last 3 Months Performance
● Special Remarks
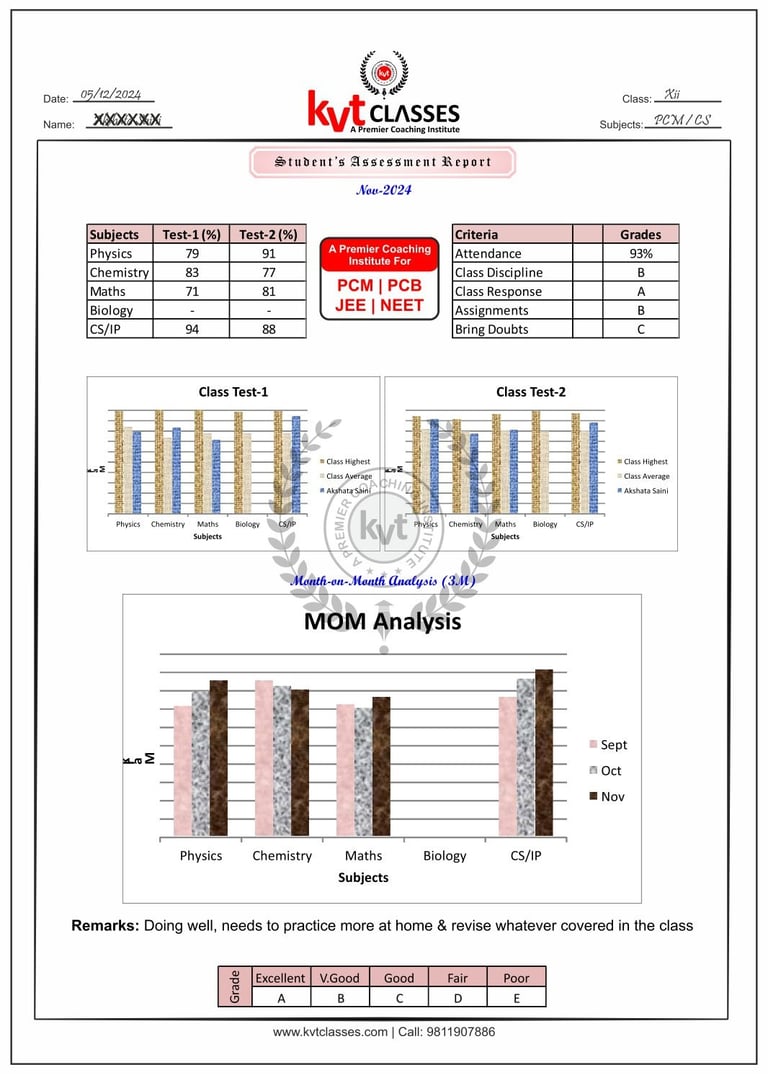
Student's Sample Report
Class Tests
● Two tests per subject in a month
● Test schedule on Saturday in extra hours
● Result within 1 week of test conduct
● Test discussion on result day
● Mistakes rectification briefing


Class-XII


Trusted by Many Prestigious School Students
Students from many prestigious Schools in the vicinity trusted KVT Classes for their Education needs because we believe in giving Quality Education. We get students all across South Delhi who study in different Schools mentioned below..


Star Performers KVT
Students who made us proud


Conduct of Classes
● On board explanation with notes
● Make Concept clear with practical examples
● Interactive class sessions
● Home work / Assignment at the end of the class
● Home work / Assignment check in the next class
● Quick recap of previous class in the next class
● Two tests per month/subject on Saturdays
● Class duration 1 Hr/sub alternate days (3Hrs./wk)
● Extra classes provided when needed
Classes Schedule 2024-25
Science Students Class XI-XII
Note: Class Tests will be conducted on Saturday in extra hours
Syllabus & Marks Weightage [ Physics ]
Syllabus & Marks Weightage [ Chemistry ]
Syllabus & Marks Weightage [ Biology ]
Detailed Syllabus [ Physics ] XII
Unit I: Electrostatics 26 Periods
Chapter–1: Electric Charges and Fields
Electric charges, Conservation of charge, Coulomb's law-force between two- point charges, forces between multiple charges; superposition principle and continuous charge distribution. Electric field, electric field due to a point charge, electric field lines, electric dipole, electric field due to a dipole, torque on a dipole in uniform electric field. Electric flux, statement of Gauss's theorem and its applications to find field due to infinitely long straight wire, uniformly charged infinite plane sheet and uniformly charged thin spherical shell (field inside and outside).
Chapter–2: Electrostatic Potential and Capacitance
Electric potential, potential difference, electric potential due to a point charge, a dipole and system of charges; equipotential surfaces, electrical potential energy of a system of two-point charges and of electric dipole in an electrostatic field. Conductors and insulators, free charges and bound charges inside a conductor. Dielectrics and electric polarization, capacitors and capacitance, combination of capacitors in series and in parallel, capacitance of a parallel plate capacitor with and without dielectric medium between the plates, energy stored in a capacitor (no derivation, formulae only).
Unit II: Current Electricity 18 Periods
Chapter–3: Current Electricity
Electric current, flow of electric charges in a metallic conductor, drift velocity, mobility and their relation with electric current; Ohm's law, V-I characteristics (linear and non-linear), electrical energy and power, electrical resistivity and conductivity, temperature dependence of resistance, Internal resistance of a cell, potential difference and emf of a cell, combination of cells in series and in parallel, Kirchhoff's rules, Wheatstone bridge.
Unit III: Magnetic Effects of Current and Magnetism 25 Periods
Chapter–4: Moving Charges and Magnetism
Concept of magnetic field, Oersted's experiment. Biot - Savart law and its application to current carrying circular loop. Ampere's law and its applications to infinitely long straight wire. Straight solenoid (only qualitative treatment), force on a moving charge in uniform magnetic and electric fields. Force on a current-carrying conductor in a uniform magnetic field, force between two parallel current-carrying conductors-definition of ampere, torque experienced by a current loop in uniform magnetic field; Current loop as a magnetic dipole and its magnetic dipole moment, moving coil galvanometer- its current sensitivity and conversion to ammeter and voltmeter.
Chapter–5: Magnetism and Matter
Bar magnet, bar magnet as an equivalent solenoid (qualitative treatment only), magnetic field intensity due to a magnetic dipole (bar magnet) along its axis and perpendicular to its axis (qualitative treatment only), torque on a magnetic dipole (bar magnet) in a uniform magnetic field (qualitative treatment only), magnetic field lines. Magnetic properties of materials- Para-, dia- and ferro - magnetic substances with examples, Magnetization of materials, effect of temperature on magnetic properties.
Unit IV: Electromagnetic Induction and Alternating Currents 24 Periods
Chapter–6: Electromagnetic Induction
Electromagnetic induction; Faraday's laws, induced EMF and current; Lenz's Law, Self and mutual induction.
Chapter–7: Alternating Current
Alternating currents, peak and RMS value of alternating current/voltage; reactance and impedance; LCR series circuit (phasors only), resonance, power in AC circuits, power factor, wattless current. AC generator, Transformer.
Unit V: Electromagnetic waves 04 Periods
Chapter–8: Electromagnetic Waves
Basic idea of displacement current, Electromagnetic waves, their characteristics, their transverse nature (qualitative idea only). Electromagnetic spectrum (radio waves, microwaves, infrared, visible, ultraviolet, X-rays, gamma rays) including elementary facts about their uses.
Unit VI: Optics 30 Periods
Chapter–9: Ray Optics and Optical Instruments
Ray Optics: Reflection of light, spherical mirrors, mirror formula, refraction of light, total internal reflection and optical fibers, refraction at spherical surfaces, lenses, thin lens formula, lens maker’s formula, magnification, power of a lens, combination of thin lenses in contact, refraction of light through a prism. Optical instruments: Microscopes and astronomical telescopes (reflecting and refracting) and their magnifying powers.
Chapter–10: Wave Optics
Wave optics: Wave front and Huygen’s principle, reflection and refraction of plane wave at a plane surface using wave fronts. Proof of laws of reflection and refraction using Huygen’s principle. Interference, Young's double slit experiment and expression for fringe width (No derivation final expression only), coherent sources and sustained interference of light, diffraction due to a single slit, width of central maxima (qualitative treatment only).
Unit VII: Dual Nature of Radiation and Matter 08 Periods
Chapter–11: Dual Nature of Radiation and Matter
Dual nature of radiation, Photoelectric effect, Hertz and Lenard's observations; Einstein's photoelectric equation-particle nature of light. Experimental study of photoelectric effect Matter waves-wave nature of particles, de-Broglie relation.
Unit VIII: Atoms and Nuclei 15 Periods
Chapter–12: Atoms
Alpha-particle scattering experiment; Rutherford's model of atom; Bohr model of hydrogen atom, Expression for radius of nth possible orbit, velocity and energy of electron in nth orbit, hydrogen line spectra (qualitative treatment only).
Chapter–13: Nuclei
Composition and size of nucleus, nuclear force Mass-energy relation, mass defect; binding energy per nucleon and its variation with mass number; nuclear fission, nuclear fusion.
Unit IX: Electronic Devices 10 Periods
Chapter–14: Semiconductor
Electronics: Materials, Devices and Simple Circuits
Energy bands in conductors, semiconductors and insulators (qualitative ideas only) Intrinsic and extrinsic semiconductors- p and n type, p-n junction Semiconductor diode - I-V characteristics in forward and reverse bias, application of junction diode -diode as a rectifier.
Detailed Syllabus [ Chemistry ] XII
Unit I: Solutions 10 Periods
Types of solutions, expression of concentration of solutions of solids in liquids, solubility of gases in liquids, solid solutions, Raoult's law, colligative properties - relative lowering of vapour pressure, elevation of boiling point, depression of freezing point, osmotic pressure, determination of molecular masses using colligative properties, abnormal molecular mass, Van't Hoff factor.
Unit II: Electrochemistry 12 Periods
Redox reactions, EMF of a cell, standard electrode potential, Nernst equation and its application to chemical cells, Relation between Gibbs energy change and EMF of a cell, conductance in electrolytic solutions, specific and molar conductivity, variations of conductivity with concentration, Kohlrausch's Law, electrolysis and law of electrolysis (elementary idea), dry cell-electrolytic cells and Galvanic cells, lead accumulator, fuel cells, corrosion.
Unit III: Chemical Kinetics 10 Periods
Rate of a reaction (Average and instantaneous), factors affecting rate of reaction: concentration, temperature, catalyst; order and molecularity of a reaction, rate law and specific rate constant, integrated rate equations and half-life (only for zero and first order reactions), concept of collision theory (elementary idea, no mathematical treatment), activation energy, Arrhenius equation.
Unit IV: d and f Block Elements 12 Periods
General introduction, electronic configuration, occurrence and characteristics of transition metals, general trends in properties of the first row transition metals – metallic character, ionization enthalpy, oxidation states, ionic radii, colour, catalytic property, magnetic properties, interstitial compounds, alloy formation, preparation and properties of K2Cr2O7 and KMnO4.
Lanthanoids - Electronic configuration, oxidation states, chemical reactivity and lanthanoid contraction and its consequences.
Actinoids - Electronic configuration, oxidation states and comparison with lanthanoids.
Unit V: Coordination Compounds 12 Periods
Coordination compounds - Introduction, ligands, coordination number, colour, magnetic properties and shapes, IUPAC nomenclature of mononuclear coordination compounds. Bonding, Werner's theory, VBT, and CFT; structure and stereoisomerism, importance of coordination compounds (in qualitative analysis, extraction of metals and biological system).
Unit VI: Haloalkanes and Haloarenes. 10 Periods
Haloalkanes: Nomenclature, nature of C–X bond, physical and chemical properties, optical rotation mechanism of substitution reactions.
Haloarenes: Nature of C–X bond, substitution reactions (Directive influence of halogen in monosubstituted compounds only).
Uses and environmental effects of - dichloromethane, trichloromethane, tetrachloromethane, iodoform, freons, DDT.
Unit VII: Alcohols, Phenols and Ethers 10 Periods
Alcohols: Nomenclature, methods of preparation, physical and chemical properties (of primary alcohols only), identification of primary, secondary and tertiary alcohols, mechanism of dehydration, uses with special reference to methanol and ethanol.
Phenols: Nomenclature, methods of preparation, physical and chemical properties, acidic nature of phenol, electrophillic substitution reactions, uses of phenols.
Ethers: Nomenclature, methods of preparation, physical and chemical properties, uses.
Unit VIII: Aldehydes, Ketones and Carboxylic Acids 10 Periods
Aldehydes and Ketones: Nomenclature, nature of carbonyl group, methods of preparation, physical and chemical properties, mechanism of nucleophilic addition, reactivity of alpha hydrogen in aldehydes, uses.
Carboxylic Acids: Nomenclature, acidic nature, methods of preparation, physical and chemical properties; uses.
Unit IX: Amines 10 Periods
Amines: Nomenclature, classification, structure, methods of preparation, physical and chemical properties, uses, identification of primary, secondary and tertiary amines.
Diazonium salts: Preparation, chemical reactions and importance in synthetic organic chemistry.
Unit X: Biomolecules 12 Periods
Carbohydrates - Classification (aldoses and ketoses), monosaccahrides (glucose and fructose), D-L configuration oligosaccharides (sucrose, lactose, maltose), polysaccharides (starch, cellulose, glycogen); Importance of carbohydrates.
Proteins -Elementary idea of - amino acids, peptide bond, polypeptides, proteins, structure of proteins - primary, secondary, tertiary structure and quaternary structures (qualitative idea only), denaturation of proteins; enzymes. Hormones - Elementary idea excluding structure.
Vitamins - Classification and functions.
Nucleic Acids: DNA and RNA.ere...
Detailed Syllabus [ Biology ] XII
Unit-VI Reproduction
Chapter-1: Sexual Reproduction in Flowering Plants
Flower structure; development of male and female gametophytes; pollination - types, agencies and examples; out breeding devices; pollen-pistil interaction; double fertilization; post fertilization events - development of endosperm and embryo, development of seed and formation of fruit; special modes- apomixis, parthenocarpy, polyembryony; Significance of seed dispersal and fruit formation.
Chapter-2: Human Reproduction
Male and female reproductive systems; microscopic anatomy of testis and ovary; gametogenesis -spermatogenesis and oogenesis; menstrual cycle; fertilisation, embryo development upto blastocyst formation, implantation; pregnancy and placenta formation (elementary idea); parturition (elementary idea); lactation (elementary idea).
Chapter-3: Reproductive Health
Need for reproductive health and prevention of Sexually Transmitted Diseases (STDs); birth control - need and methods, contraception and medical termination of pregnancy (MTP); amniocentesis; infertility and assisted reproductive technologies - IVF, ZIFT, GIFT (elementary idea for general awareness).
Unit-VII Genetics and Evolution
Chapter-4: Principles of Inheritance and Variation
Heredity and variation: Mendelian inheritance; deviations from Mendelism – incomplete dominance, co-dominance, multiple alleles and inheritance of blood groups, pleiotropy; elementary idea of polygenic inheritance; chromosome theory of inheritance; chromosomes and genes; Sex determination - in humans, birds and honey bee; linkage and crossing over; sex linked inheritance - haemophilia, colour blindness; Mendelian disorders in humans - thalassemia; chromosomal disorders in humans; Down's syndrome, Turner's and Klinefelter's syndromes.
Chapter-5: Molecular Basis of Inheritance
Search for genetic material and DNA as genetic material; Structure of DNA and RNA; DNA packaging; DNA replication; Central Dogma; transcription, genetic code, translation; gene expression and regulation - lac operon; Genome, Human and rice genome projects; DNA fingerprinting.
Chapter-6: Evolution
Origin of life; biological evolution and evidences for biological evolution (paleontology, comparative anatomy, embryology and molecular evidences); Darwin's contribution, modern synthetic theory of evolution; mechanism of evolution - variation (mutation and recombination) and natural selection with examples, types of natural selection; Gene flow and genetic drift; Hardy- Weinberg's principle; adaptive radiation; human evolution.
Unit-VIII: Biology and Human Welfare
Chapter-7: Human Health and Diseases
Pathogens; parasites causing human diseases (malaria, dengue, chikungunya, filariasis, ascariasis, typhoid, pneumonia, common cold, amoebiasis, ring worm) and their control; Basic concepts of immunology - vaccines; cancer, HIV and AIDS; Adolescence - drug and alcohol abuse.
Chapter-8: Microbes in Human Welfare
Microbes in food processing, industrial production, sewage treatment, energy generation and microbes as bio-control agents and bio-fertilizers. Antibiotics; production and judicioususe.
Unit-IX Biotechnology and its Applications
Chapter-9: Biotechnology - Principles and Processes
Genetic Engineering (Recombinant DNA Technology).
Chapter-10: Biotechnology and its Applications
Application of biotechnology in health and agriculture: Human insulin and vaccine production, stem cell technology, gene therapy; genetically modified organisms - Bt crops; transgenic animals; biosafety issues, biopiracy and patents.
Unit-X Ecology and Environment
Chapter-11: Organisms and Populations
Population interactions - mutualism, competition, predation, parasitism; population attributes - growth, birth rate and death rate, age distribution. (Topics excluded: Organism and its Environment, Major Aboitic Factors, Responses to Abioitic Factors, Adaptations)
Chapter-12: Ecosystem
Ecosystems: Patterns, components; productivity and decomposition; energy flow; pyramids of number, biomass, energy (Topics excluded: Ecological Succession and Nutrient Cycles).
Chapter-13: Biodiversity and its Conservation
Biodiversity-Concept, patterns, importance; loss of biodiversity; biodiversity conservation; hotspots, endangered organisms, extinction, Red Data Book, Sacred Groves, biosphere reserves, national parks, wildlife, sanctuaries and Ramsar sites.
